Seed Funding Program 2022
VITAL
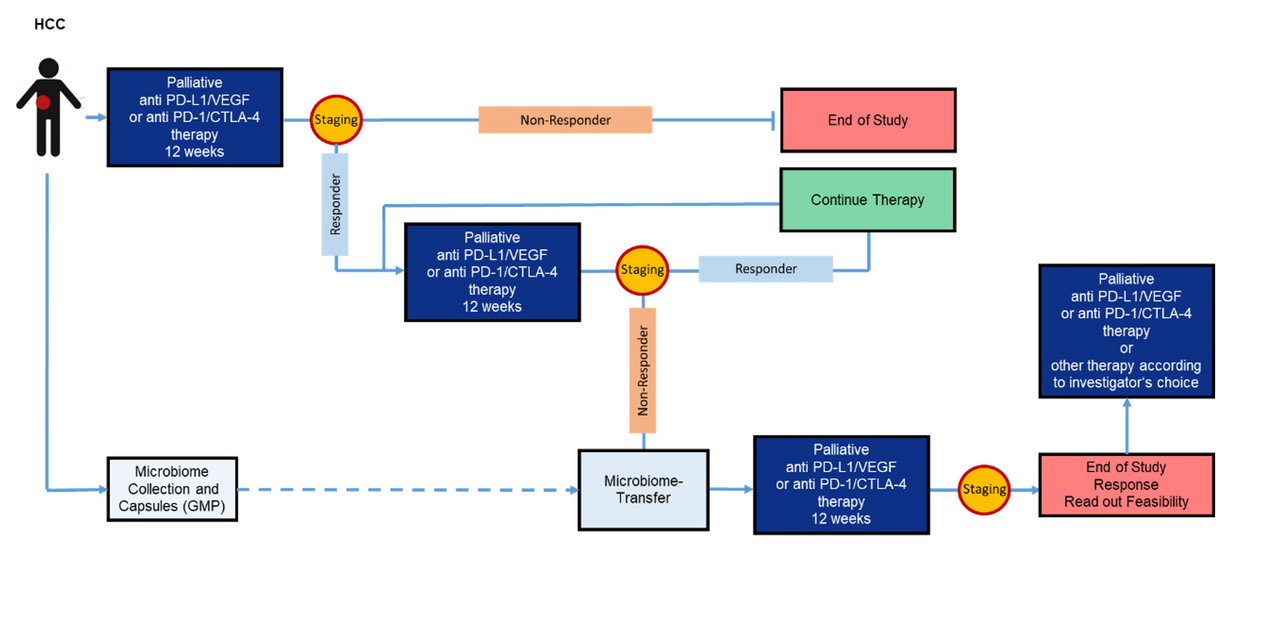
A pilot and feasibility trial for evaluation of PD-L1 inhibitor Atezolizumab & VEGF Inhibitor Bevacizumab treatment combined with autologous fecal microbiota transfer for patients with advanced liver cancer
Coordinators
 dkfz.de
dkfz.de
Matthias Ebert
II. Medical Clinic, UMM

Eran Elinav
Microbiome and Cancer, DKFZ
Detailed description
The recent IMbrave150 trial showed that immunotherapy with Atezolizumab plus Bevacizumab (A&B) improved overall survival in patients with hepatocellular carcinoma. Gut microbiota are critical for response to checkpoint inhibitors and recent reports indicate that responder-derived fecal microbiota transplantation (FMT) is effective to overcome PD-1 resistance in melanoma patients. The pilot trial aims to assess the feasibility of a new therapy concept with A&B in hepatocellular carcinoma in combination with autologous FMT in responders at the time of progressive disease. It will test the feasibility of potentially reversing secondary immunotherapy resistance after A&B by FMT. The project serves for developing the trial protocol and assessing the feasibility of the translational program in a pilot trial. The trial protocol will also address new treatment options with double checkpoint blockade (PD-1/CTLA-4) which was recently also approved for liver cancer therapy.