Seed Funding Program 2023
SMAD-PC
SMAD4 loss remodels phosphatidylcholine metabolism in colorectal cancer
Coordinators

Sebastian Schölch
DKFZ Hector Junior Clinical Cooperation Unit Translational Surgical Oncology, UMM

Almut Schulze
Tumour Metabolism and Microenvironment, DKFZ
Detailed description
Colorectal cancer remains a critical health concern with extensive global ramifications. Despite substantial advancements in our comprehension of its genetic causes, particularly the adenoma-carcinoma sequence, notable gaps persist in our knowledge. The tumor suppressor gene SMAD4 is frequently mutated in colorectal cancer and canonically associated with perturbations in the TGF-β signaling pathway. Our data indicates that the loss of SMAD4 also triggers significant transcriptomic and proteomic alterations that extend beyond the traditional TGF-β pathway, suggesting modifications in cellular membrane microdomains, which appear to confer substantial survival benefits to SMAD4-mutant cancer cells.
Utilizing a multifaceted approach that includes preclinical models, patient-derived organoid lines, CRISPR-Cas9 genome-editing and mass spectrometry, this project aims to elucidate the detailed mechanisms underpinning these non-canonical pathways, aiming to identify novel therapeutic targets and develop innovative treatment strategies specifically for SMAD4-mutant colorectal cancer
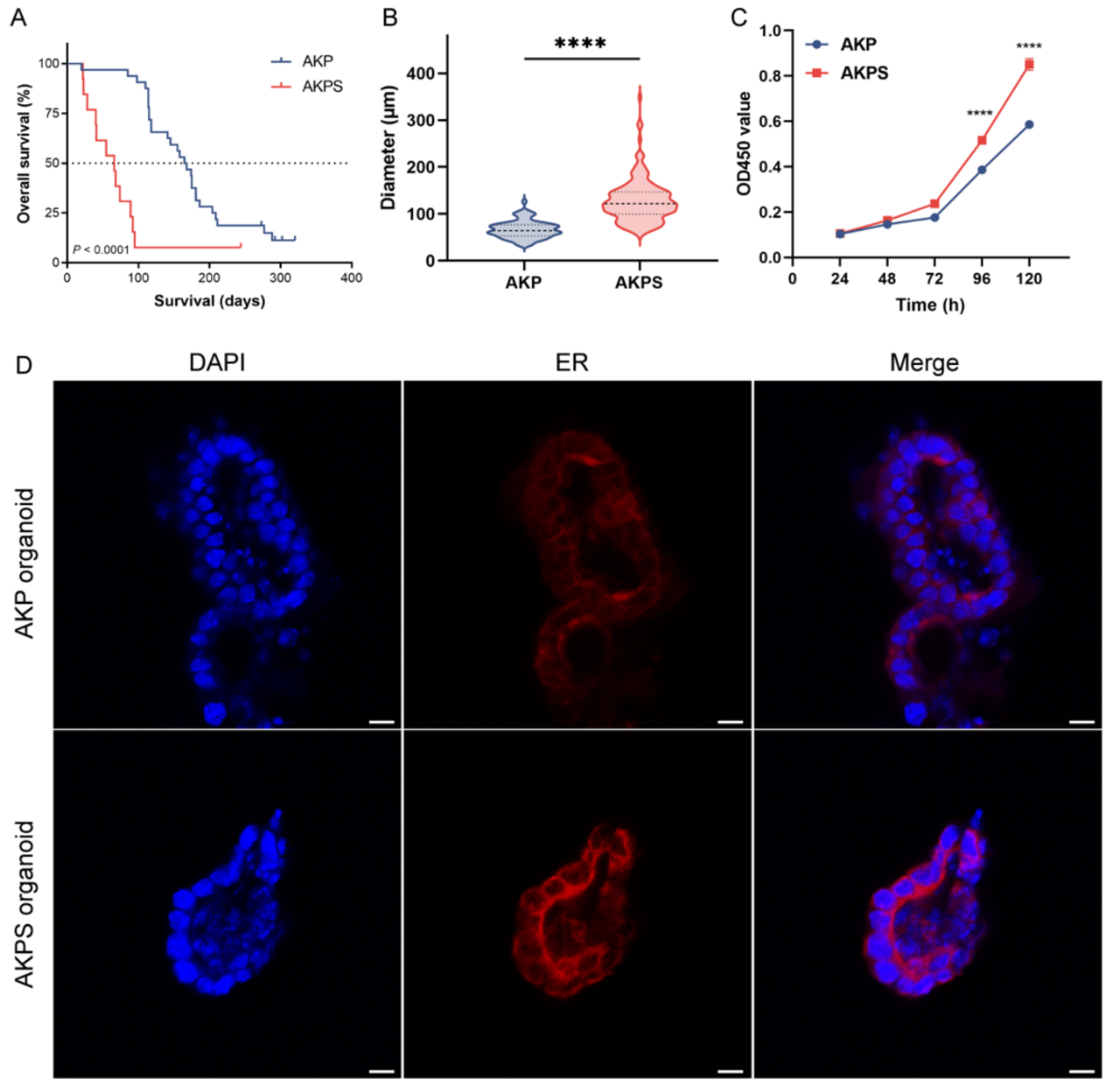
Illustration: A. Smad4 mutations lead to significantly poorer survival in preclinical colorectal cancer models. B/C. Smad4 mutations increase the size (B) and proliferation activity (C) of murine colorectal cancer organoids. D. The altered membrane metabolism is evident in the endoplasmic reticulum.